FEATURED POST
Quality Engineering
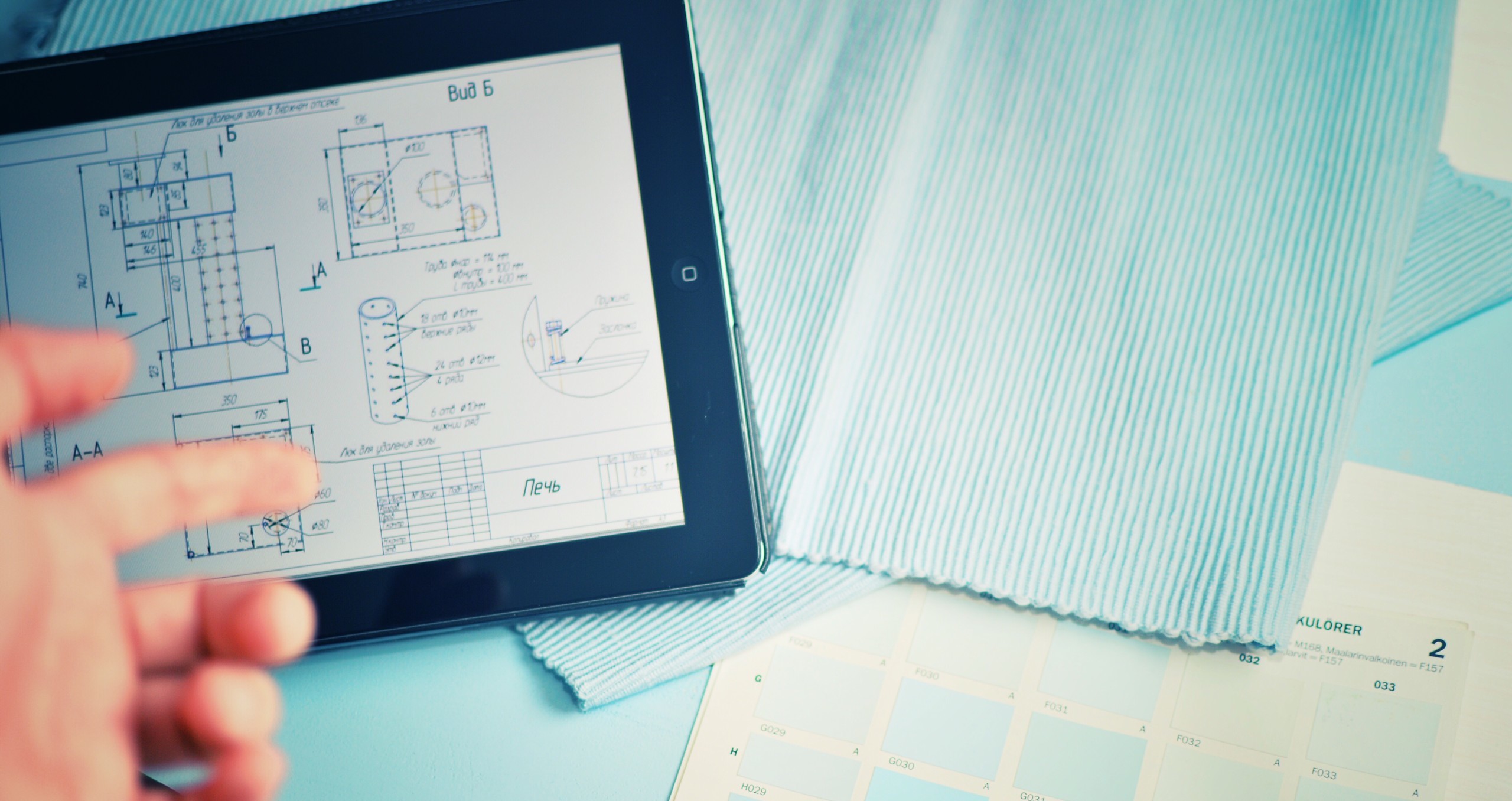
Are you able to resolve the root cause?
Quality Engineering services using proven methodologies to resolve life science engineering challenges
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory support for all phases [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare for your next FDA [...]
TEAM MEMBER PROFILE Meet Ruben Capo: Co-Founder, Quality Engineer, Part-Time Adventurer
Meet Ruben, mild-mannered BioTeknica Principal, Co-Founder, and Senior Quality Engineer. Think you know him? Think again. When you [...]
INDUSTRY NEWS ROUNDUP
Due to the COVID-19 pandemic, the Association of Food and Drug Officials (AFDO) Annual Conference, originally scheduled for June [...]
SUCCESS STORIES Bringing an Inadequate CAPA Process to Effectiveness
Did you know that one of the top three causes of 483 observations is because manufacturers fail to correctly [...]
ASK A BIOTEKNICA SME HOW TO VALIDATE A PROCESS WITH A “HIGH DEGREE OF ASSURANCE” AND WHAT DOES THAT EVEN MEAN?
Looking for answers to your regulatory questions? To submit one, please click on Ask a BioTeknica SME and our Subject Matter [...]
IS/IT Information Systems Management
IS/ IT Information Systems Management A Division of BioTeknica, an IQVIA Business About Our Information Systems Management Services [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare [...]
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015 BioTeknica has successfully achieved ISO 9001:2015 certification, the highest standards of its kind, for its Quality Management (QM) [...]
Ready, Set, Go! Coaching Your Team to Validation Success
Ready, Set, Go! Coaching Your Team to Validation Success Device manufacturers often don’t have the time or the internal resources to train their [...]
Get to the Head of the Class: Lunch & Learn Series at BioTeknica’s New Learning Center
Get to the Head of the Class: Lunch & Learn Series at BioTeknica's New Learning Center BioTeknica recently launched Lunch & Learn, a [...]
Julie Larsen Joins FDANews Medical Device Conference Advisory Board
Julie Larsen Joins FDANews Medical Device Conference Advisory Board Congratulations to BioTeknica principal Julie Larsen for her recent invitation and acceptance to join [...]
Charity: BioTeknica’s Helping Hands
Charity: BioTeknica's Helping Hands Every year for the past three and a half years, BioTeknica has partnered with Catholic Relief Services (CRS) to support Helping [...]
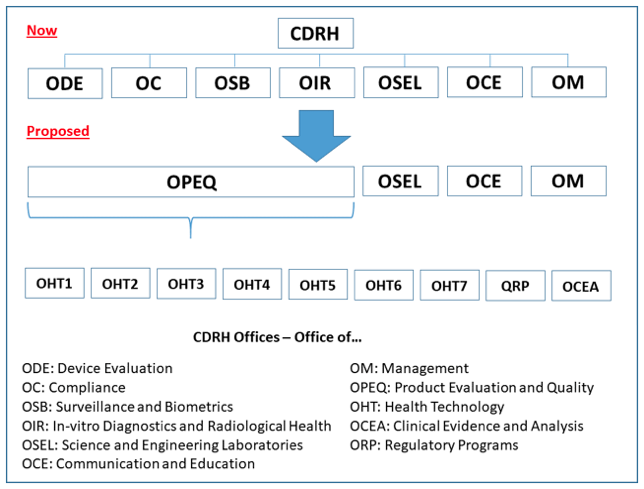
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health (CDRH) has submitted a reorganization plan [...]