FDA’s Office Of Regulatory Affairs Realigned
The FDA’s Office of Regulatory Affairs (ORA), the lead office for agency field activities, has changed its management structure to align its staff by FDA-regulated product instead of geographic region. At a symposium earlier this year, an FDA official provided an update of key changes at the ORA and discussed two laws that will soon impact medical device companies.
New Organization Structure
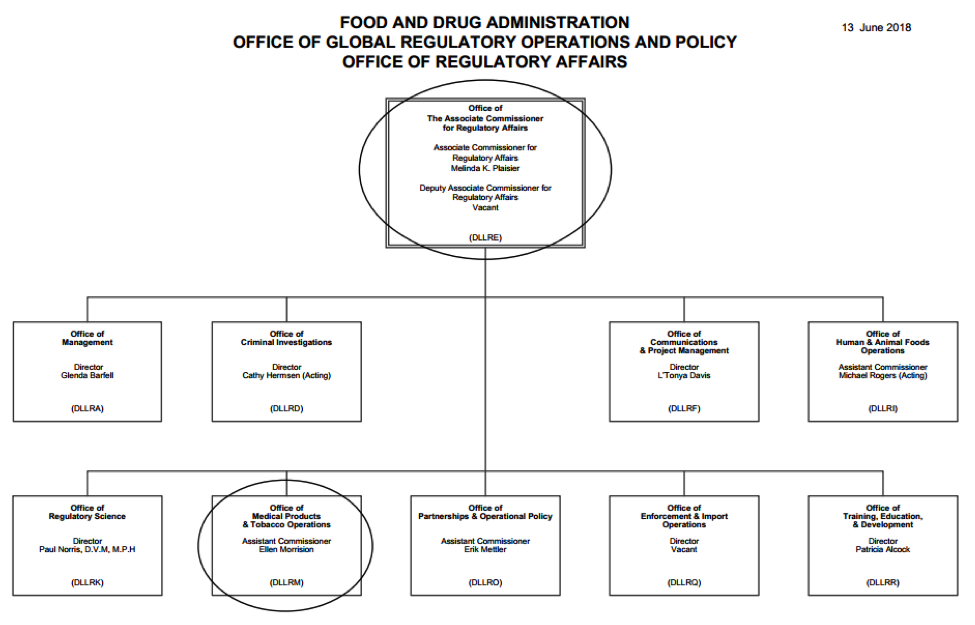
The ORA’s Office of Medical Products and Tobacco Operations (OMPTO), headed by Ellen Morrison, oversees five different Program Areas based on specific commodities – i.e., Tobacco, Biological Products, Bioresearch Monitoring, Medical Device and Radiological Health, and Pharmaceutical operations. According to the FDA, specializing by product type leverages the expertise of its staff and more closely mirrors the organizational model of its centers and the industries they regulate.
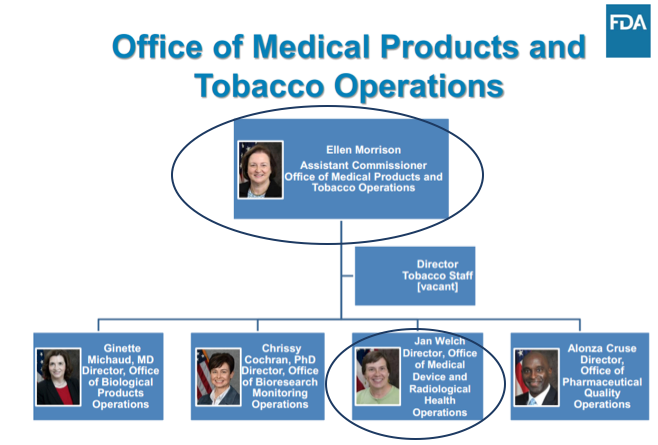
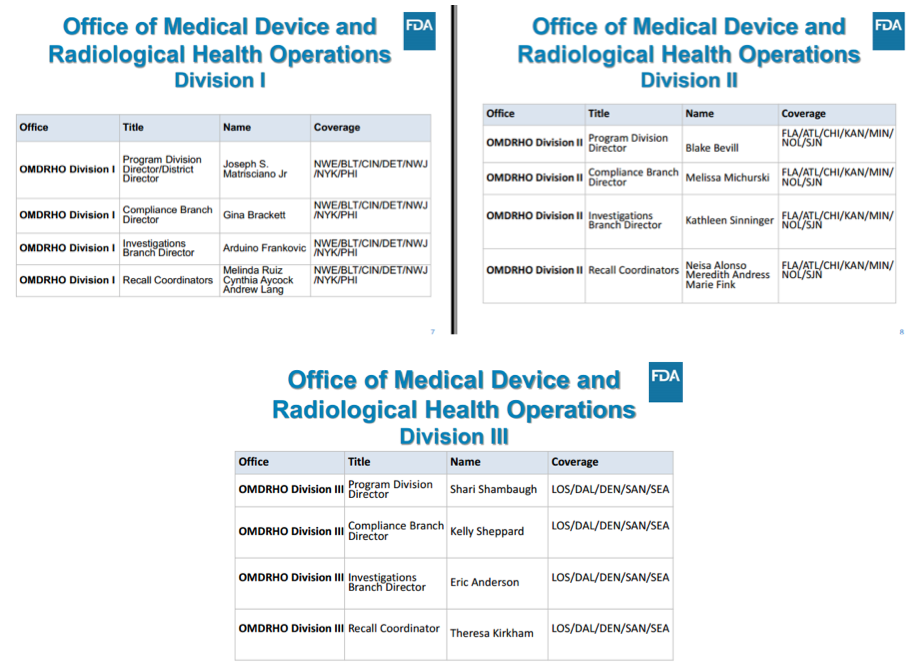
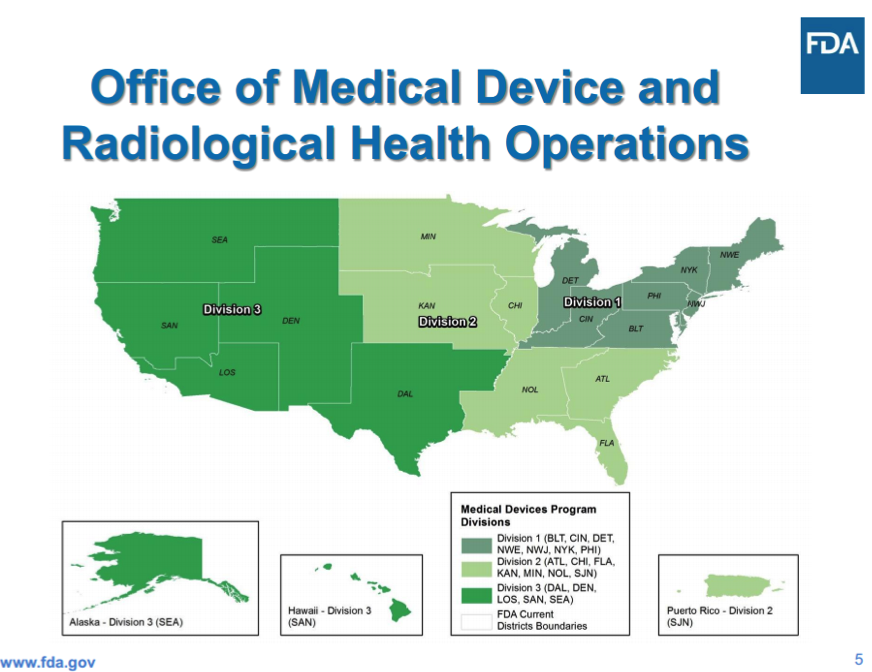
OMPTO’s Office of Medical Device and Radiological Health Operations (OMDRHO) is headed by Jan Welch, who oversees three divisions (see Boundary Map below), each headed by a Program Division Director who manages all inspections and compliance activities. Each director also oversees a Compliance Branch Director, who manages 483 responses and post-inspection compliance activities, an Investigations Branch Director, who manages all inspections activities, and a Recall Coordinator(s).
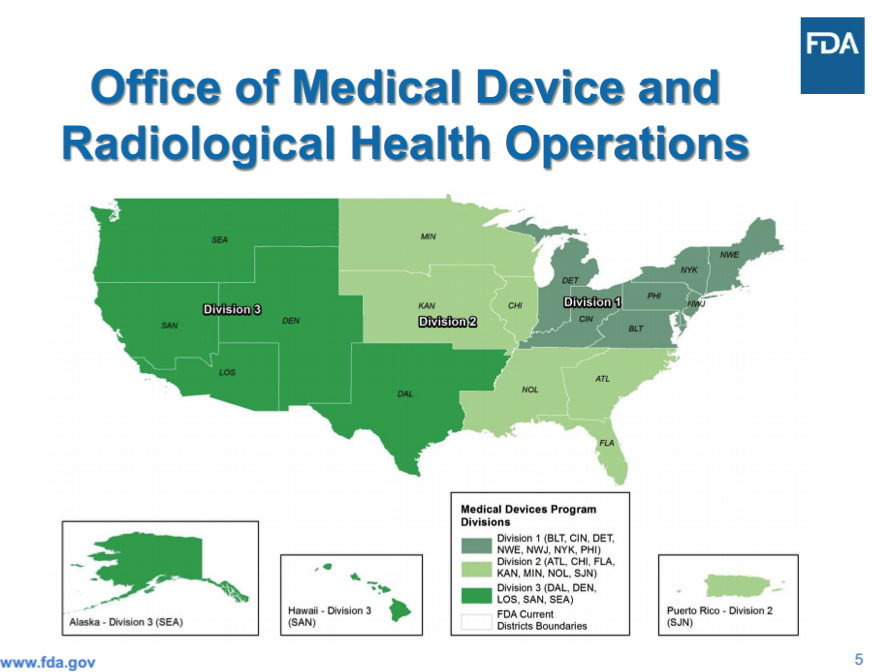
As a result of these changes, device manufacturers now have new FDA contacts depending on where they are located.



Leave A Comment