FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
The FDA Center for Devices and Radiological Health (CDRH) has submitted a reorganization plan to the agency for review, and the new program model is designed for greater inspection and approval efficiency. The realignment will impact how medical device companies interact with the agency. Find out how the agency thinks device manufacturers will benefit from these changes.
Brief History
In 2013, the FDA formed the Program Alignment Group, which was assigned to modify the FDA’s structure and processes to meet the challenges brought on by such factors as innovation and globalization. Over the past two years many of these modifications have been implemented.
Each center, including the Center for Devices and Radiological Health (CDRH), developed an action plan and goals to accomplish within the next five years. Fast forward to 2018, and this realignment is changing the focus of FDA staff from geography-based to commodity-based teams.
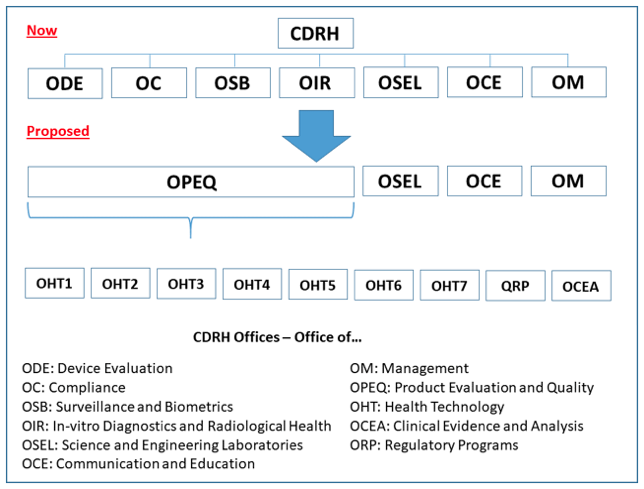
Realignment
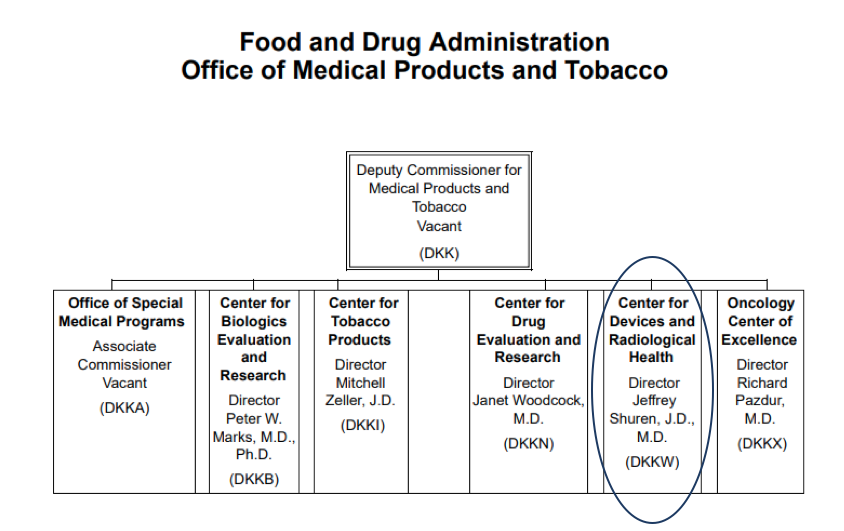
The CDRH falls within the auspices of the Office of Medical Products and Tobacco Operations. Jeffrey Shuren, JD, MD, is the Director of the CDRH.
At an industry conference earlier this year, CDRH Office of Compliance Regulatory Affairs Deputy Director Captain Sean Boyd provided important information:
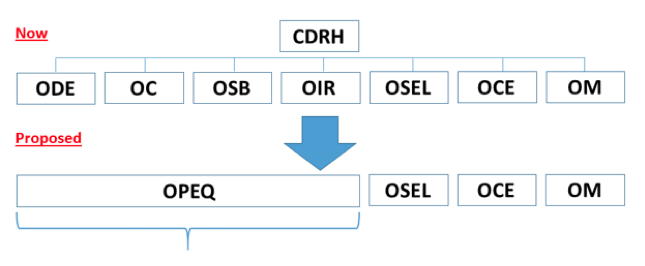
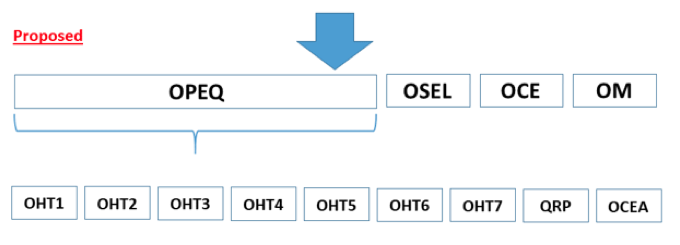
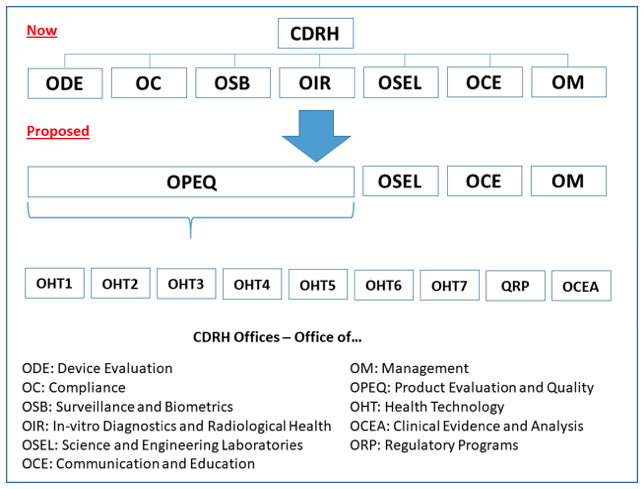
Impact on Device Manufacturers
Captain Boyd envisions a one-stop shop for regulatory and compliance information where teams can amass specific areas of knowledge and expertise not only on the technologies that are brought to market but also on subsets of these technologies, as well as understanding the development, design, performance, and testing of the devices.
The CDRH’s reorganization may benefit large and small companies because it will allow manufacturers to get to know their CDRH teams and ultimately enhance communication between the agency and industry. The CDRH will also look at internal and external data to identify trends across multiple OHTs that may also be found industrywide. In other words, they may find trends among multiple companies that impact a specific area or find trends for a specific manufacturer that applies to multiple therapeutic areas.
The Total Product Life Cycle database, available to the public, provides information on premarket and post-market data (e.g., PMAs, 510ks, Adverse Events, Recalls). Updated frequently, the database can be searched by device name, product code, or generic category.



Leave A Comment