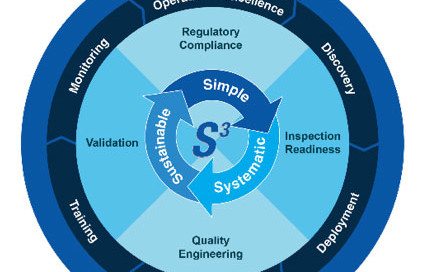
FDA & EU Requirements and Expectations
5 Things You Should Know from the 2015 Association of Food & Drug Officials (AFDO) Conference If you didn’t have a chance to attend this year’s AFDO Annual Educational Conference, below are five important take-away points from the Drugs, Devices and Cosmetics sessions. 1. EU Enforcement: Notified Bodies Now Conducting Unannounced Audits of Manufacturers, [...]