FEATURED POST
Quality Engineering
Are you able to resolve the root cause?
Quality Engineering services using proven methodologies to resolve life science engineering challenges
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory support for all phases [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare for your next FDA [...]
BIOTEKNICA COMMUNITY OUTREACH
BIOTEKNICA COMMUNITY OUTREACH Peru, Miami, Africa! When not traveling around the world for client business, BioTeknica team members can [...]
TEAM MEMBER PROFILE Meet Ruben Capo: Co-Founder, Quality Engineer, Part-Time Adventurer
Meet Ruben, mild-mannered BioTeknica Principal, Co-Founder, and Senior Quality Engineer. Think you know him? Think again. When you [...]
INDUSTRY NEWS ROUNDUP
Due to the COVID-19 pandemic, the Association of Food and Drug Officials (AFDO) Annual Conference, originally scheduled for June [...]
SUCCESS STORIES Bringing an Inadequate CAPA Process to Effectiveness
Did you know that one of the top three causes of 483 observations is because manufacturers fail to correctly [...]
Technical Development Service
Medical Device Design Control, Product Development & Quality Testing Product Development Management Design Control Process/Design and Implementation Regulatory Compliance Regulatory, Quality & Clinical Trial [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact the device industry: [...]
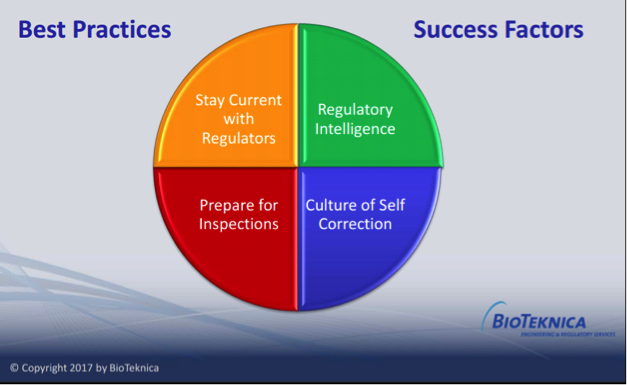
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American Society for Quality (ASQ) conference [...]
Validation: When is a Test Method Not a Test Method?
Validation: When is a Test Method Not a Test Method? How can one site conduct and pass several gage Repeatability and Reproducibility (R&R) [...]
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015 BioTeknica has successfully achieved ISO 9001:2015 certification, the highest standards of its kind, for its Quality Management (QM) [...]
Ready, Set, Go! Coaching Your Team to Validation Success
Ready, Set, Go! Coaching Your Team to Validation Success Device manufacturers often don’t have the time or the internal resources to train their [...]
Get to the Head of the Class: Lunch & Learn Series at BioTeknica’s New Learning Center
Get to the Head of the Class: Lunch & Learn Series at BioTeknica's New Learning Center BioTeknica recently launched Lunch & Learn, a [...]