At a half-day conference of the American Society for Quality (ASQ) conference whose theme was Quality is Not a Goal – It’s a Way of Life, Julie Larsen provided industry professionals essential tips and best practices to stay current as compliance trends evolve.
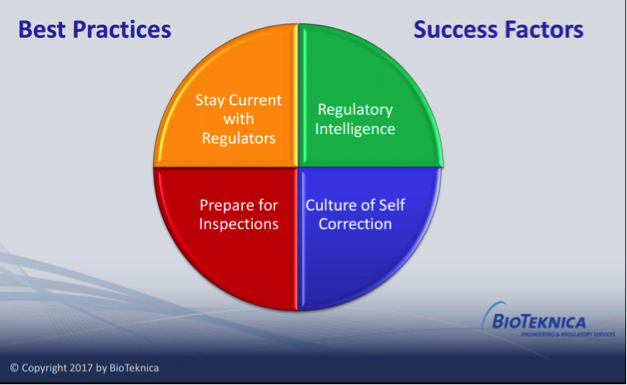
In her talk, “Keeping Current in an Evolving World of Compliance,” BioTeknica Principal and Director of Inspection Readiness Julie Larsen identified four strategies for staying current with evolving trends and maintaining compliance:
1. Stay Current with Regulators by adopting Quality Metrics programs, understanding the FDA’s program alignment and inspection initiatives.
2. Conduct Regulatory Intelligence by gathering, analyzing, and interpreting public regulatory information and using it to make timely data-driven decisions, proactively monitoring the current regulatory environment for opportunities to shape future regulations, guidance, policy, and legislation.
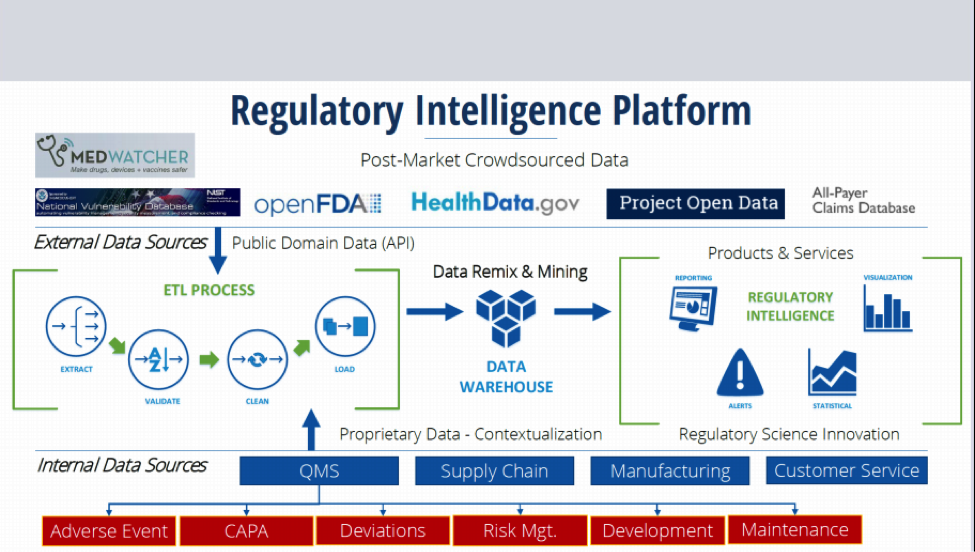
Pre-Production, Production and Distribution Data
3. Prepare for Inspections by using a simulated inspection preparation process to identify, rate, and address risk on multiple levels; for example:
a. System – where is your quality system vulnerable?
b. Subject Matter Experts – how will your SMEs perform?
c. Support Process – how will your support process hold up?
4. Embrace a Culture of Self–Correction by implementing a strong internal audit program where root causes are identified, and actions are taken, through ongoing communication with management and by retaining independent third parties to identify vulnerabilities and implement corrective actions.
Julie reviewed common mistakes made by device manufacturers:
• Failure to keep up with new requirements
• Failure to review and understand current enforcement trends
• Failure to self-identify compliance gaps
• Inadequate action for associated risk
• Complacency because of past success
Device manufacturers need to figure out how to link compliance trends to inspection preparation. Questions to ask:
• Where has the bar been raised?
• How may this apply to us?
• Where do we have similar findings?
Device manufacturers often set themselves up for failure by:
• Not addressing significant internal audit findings
• Not having CAPA subsystems in place to investigate and prevent product and quality problems
• Thinking that an investigator “would never find this”
• Assuming the company won’t be inspected because it is not located in the U.S.
• Presuming that SMEs are prepared
Some of the most common mistakes manufacturers make during an inspection include:
• Failure to:
— control documents brought into the inspection room
— verify the request was filled correctly
— review the document for unknown issues
— understand the direction and intent of the investigator/inspection
• Lack of a clearly defined inspection support process (e.g., roles, responsibilities, decision-making, etc.)
• Inability to provide data in an electronic format in a timely manner
• Inability to fill requests in a timely manner
There are other useful aids:
• Scorecards for compliance and SME performance;
• Tracking sheets to organize prep topics and records; and
• The services of an independent third party to identify vulnerabilities, compare them with current trends, and implement solutions
These are all tools that are available to the device manufacturer who wants to stay current in an evolving world of compliance.





Leave A Comment