Feeling unsure of how the FDA’s updated reporting processes will affect you? You’re not alone. BioTeknica SMEs delved into the real-world impact of these new reporting programs at a recent conference. Click below to get their Top 5 MDR tips.

Please be assured that in the event of further disruptions, we are prepared to continue with our services remotely in a secure environment for our employees and customers.
The FDA ended the Alternative Summary Reporting (ASR) program in 2019 and is now transitioning to the Voluntary Malfunction Summary Reporting (VMSR) program. Where does this leave medical device manufacturers?
Medical device reporting (MDR) regulations in both the United States and the European Union (EU) require that post-market adverse events relating to medical devices be reported and tracked. In a presentation at FDANews, BioTeknica SMEs Julie Larsen, Principal Consultant & Director of Inspection Readiness Services, and Lenita Sims-Spears, Esq., Senior QARA Consultant, were selected to speak about “Transparency and the New Medical Device Reporting Rules: What to Do Now That ASR Is Really Over.”
They provide valuable insights to medical device manufacturers.
Top 5 MDR Questions in Bite-Sized Bullets
1. What Is the ASR and Why Was it Changed?
The ASR was an FDA report and tracking program initially started in 1997 to allow the agency to efficiently review reports of well-known and well-understood adverse events. It allowed some manufacturers of certain medical devices to request an exemption from the requirement to file individual medical device reports and provide the agency with quarterly summary reports of adverse events instead of for events that were well-known and well-established risks associated with specific devices. Most of the alternative summary reports were not available through the public device reports database, MAUDE (Manufacturer and User Facility Device Experience).
Over the last two plus decades, 108 exemptions were granted to many manufacturers. To streamline medical device reporting and increase transparency, the FDA phased out ASR in June 2019 and revoked all exemptions. To address concerns that information on safety from the summary reports was not available, FDA has recently posted a comprehensive set of the ASR data (from 1997 to 2019) on its website.
2. What’s VMSR and Which Devices and Malfunctions Are Ineligible for Summary Reporting?
Manufacturers are required to submit an individual MDR for every event in which a device has caused or contributed to a death or serious patient injury or has malfunctioned and would be likely to cause or contribute to a death or serious injury should it recur. Established in 2018, the VMSR program provides real-world data from low- and medium-risk (Class I and Class II) medical devices. It enables the agency to efficiently detect potential safety signals and free up resources to better focus on the highest risks associated with devices. Through VMSR, manufacturers report certain device malfunction MDRs in summary format on a quarterly basis instead of individually for every event. The VMSR is available in the MAUDE database.
Eligible – Manufacturers of Class I and Class II devices that have product codes in existence for 2+ years can self-select to participate in VMSR. These include devices that are NOT:
- Permanently implantable
- Life supporting
- Life sustaining
Eligible product codes were determined by the FDA based on a list proposed by industry representatives. Manufacturers can determine eligibility by checking the FDA’s Product Code Classification.
Ineligible – Events outside the VMSR program:
- VMSR does not apply to reportable death or serious injury events
- Participating manufacturers must submit individual MDR reports when a death or serious injury event occurs
- 5-day reporting requirements apply
3. Can an Eligible Manufacturer Still Submit an Individual Report?
There are five individual reporting conditions excluded from VMSR:
- Reportable malfunctions associated with a 5-day report
- Reportable malfunctions associated with a recall
- Reportable malfunctions associated with a public health issue
- FDA determines the manufacturer may not report in summary format
- New type of reportable malfunction occurs for a device
4. What Is the VMSR Submission Schedule?
- Eligible manufacturers must send the summary report of all adverse events (excluding death or serious injury) on a quarterly basis. See table below:
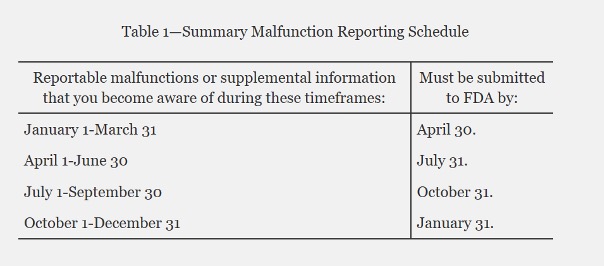
- The summary report is submitted electronically
- The information manufacturers provide needs to be current as of the last date of the quarter
5. What is the National Evaluation System for Health Technology (NEST) and How Will the FDA Monitor Medical Device Performance through It?
NEST is a national surveillance system that allows the FDA to obtain real-world evidence across the total product lifecycle of medical devices. The agency is collaborating with stakeholders, including industry, patient, and clinician groups, payers, and health systems, to actively monitor device performance and patient safety. Information will be derived from data relating to patient health status and/or data from point-of-care (Real-World Data – RWD) and clinical evidence (Real-World Evidence) about the usage and potential risks/benefits of a product derived from RWD. The program will be governed by the NEST Coordinating Center (NESTcc), which oversees infrastructure building, promotes standards, and monitors progress.
If you have any specific questions regarding ASR, VMSR, or NEST, please contact Julie Larsen by clicking on this link ASR, VMSR & NEST Questions.



Leave A Comment