FEATURED POST
What You Can Learn from 7 Theme Fusion Success Stories
Nam lacinia arcu tortor, nec luctus nibh dignissim eu. Nulla sit amet maximus nulla. Pellentesque a accumsan eros, ac molestie nulla. Morbi interdum in neque vitae vulputate.
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We ensure that your quality [...]
Lean Quality Solutions
Lean Quality Solutions for Medical Device & Pharmaceutical Companies Synergis, a sister company of BioTeknica, offers comprehensive lean quality solutions. [...]
BIOTEKNICA COMMUNITY OUTREACH
BIOTEKNICA COMMUNITY OUTREACH Peru, Miami, Africa! When not traveling around the world for client business, BioTeknica team members can be found [...]
ASK A BIOTEKNICA SME HOW TO VALIDATE A PROCESS WITH A “HIGH DEGREE OF ASSURANCE” AND WHAT DOES THAT EVEN MEAN?
Looking for answers to your regulatory questions? To submit one, please click on Ask a BioTeknica SME and our Subject Matter [...]
ASR IS OVER! HOW DO VMSR & NEST IMPACT YOUR ORGANIZATION?
Feeling unsure of how the FDA’s updated reporting processes will affect you? You’re not alone. BioTeknica SMEs delved into [...]
KEY FDA EXPECTATIONS FOR ADVERSE EVENT REPORTING DURING A PANDEMIC
The FDA has modified the regulations regarding adverse event reporting during a pandemic to include COVID-19. Click below for [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open and operating [...]
Operational Review
Operational Review & Optimization for Medical Device and Pharmaceutical Companies Cost Reduction Strategies Business Process Re-engineering (BPR) Process Optimization/Improvement Process Control and Automation [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We [...]
Lean Quality Solutions
Lean Quality Solutions for Medical Device & Pharmaceutical Companies Synergis, a sister company of BioTeknica, offers [...]
BIOTEKNICA COMMUNITY OUTREACH
BIOTEKNICA COMMUNITY OUTREACH Peru, Miami, Africa! When not traveling around the world for client business, BioTeknica team [...]
Get to the Head of the Class: Lunch & Learn Series at BioTeknica’s New Learning Center
Get to the Head of the Class: Lunch & Learn Series at BioTeknica's New Learning Center BioTeknica recently launched Lunch & Learn, a [...]
Julie Larsen Joins FDANews Medical Device Conference Advisory Board
Julie Larsen Joins FDANews Medical Device Conference Advisory Board Congratulations to BioTeknica principal Julie Larsen for her recent invitation and acceptance to join [...]
Upcoming Events
Upcoming Events BioTeknica will be participating in a series of events and conferences. Please see our end-of–year calendar of events.
Promotion Announcement
Promotion Announcement We are pleased to announce the promotion of several BioTeknica team members. Please join us in congratulating them all! Brian Dawson has [...]
Charity: BioTeknica’s Helping Hands
Charity: BioTeknica's Helping Hands Every year for the past three and a half years, BioTeknica has partnered with Catholic Relief Services (CRS) to support Helping [...]
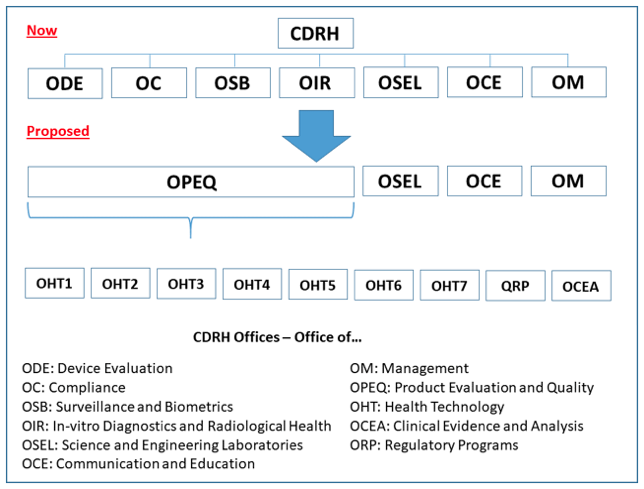
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health (CDRH) has submitted a reorganization plan [...]