FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
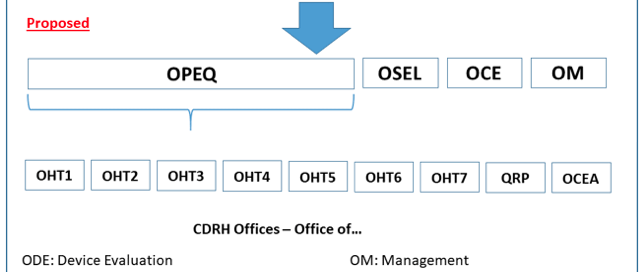
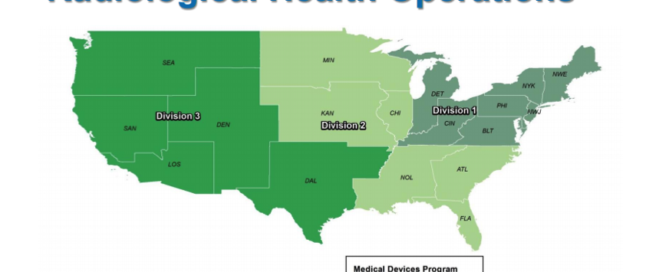
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health (CDRH) has submitted a reorganization plan to the agency for review, and the new program model is designed for greater inspection and approval efficiency. The realignment will impact how medical device companies interact with the agency. Find out how the agency thinks device manufacturers will benefit from [...]