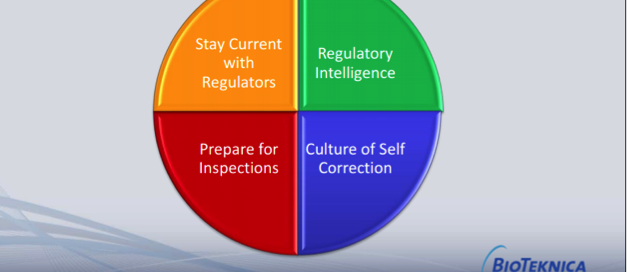
KEY FDA EXPECTATIONS FOR ADVERSE EVENT REPORTING DURING A PANDEMIC
The FDA has modified the regulations regarding adverse event reporting during a pandemic to include COVID-19. Click below for key points about the updated guidance as of March 2020. Please be assured that in the event of further disruptions, we are prepared to continue with our services remotely in a [...]