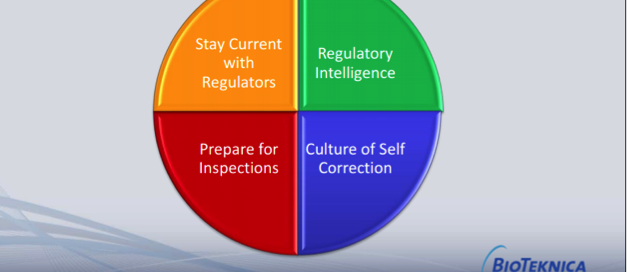
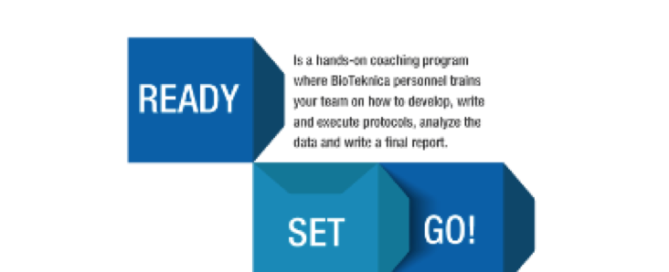
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact the device industry: 1. Food and Drug Administration Re-Authorization Act (FDARA) of 2017 2. 21st Century Cures Act FDARA Highlights The FDA Reauthorization Act of 2017 (FDARA), an amendment to the Federal Food, Drug, and [...]