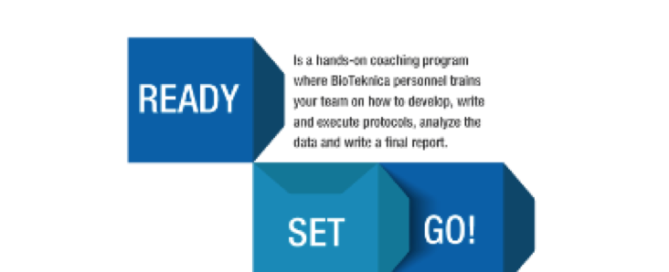
Ready, Set, Go! Coaching Your Team to Validation Success
Ready, Set, Go! Coaching Your Team to Validation Success Device manufacturers often don’t have the time or the internal resources to train their staff. BioTeknica’s Ready Set Go! is a proprietary, interactive, hands-on coaching program where our personnel help you successfully train your team members in developing and executing validation protocols, performing test result analysis, and [...]