Validation
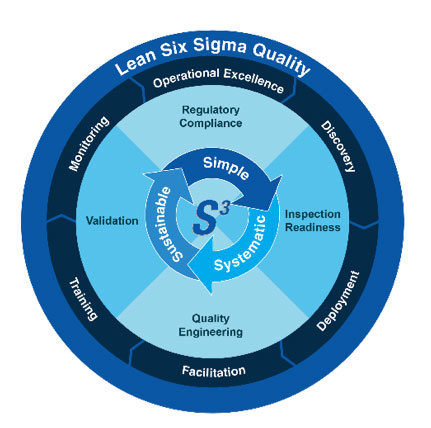
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both small and large Medical Device and Pharmaceutical manufacturers. Validation Services for Medical Device & Pharmaceutical Manufacturers Do all of your products, equipment, processes, software, and systems operate as designed and intended? Inadequate validation is a common source of FDA 483 findings. Responding to them can be costly and disruptive, especially if it requires the validation of your legacy products, test methods, equipment, and processes. If just one validation segment doesn’t meet regulatory standards, it can negatively impact your organization. We enhance your future bottom line by characterizing, optimizing, and thoroughly validating your production processes and systems. Validation [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open and operating at full [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact [...]
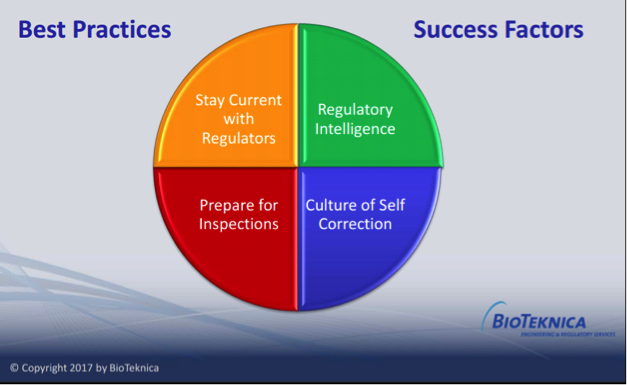
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American Society for [...]
Promotion Announcement
Promotion Announcement We are pleased to announce the promotion of several BioTeknica team members. Please join us in [...]
Charity: BioTeknica’s Helping Hands
Charity: BioTeknica's Helping Hands Every year for the past three and a half years, BioTeknica has partnered with Catholic Relief [...]
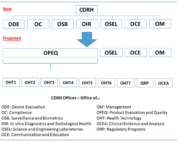
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health [...]
FDA’s Office Of Regulatory Affairs Realigned
FDA's Office Of Regulatory Affairs Realigned The FDA’s Office of Regulatory Affairs (ORA), the lead office for agency [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both small and [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two [...]
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of [...]
Successful Outcomes One Client at a Time
Successful Outcomes One Client at a Time Recently, BioTeknica assisted a leading medical device manufacturer to successfully address a previous FDA Warning Letter by [...]
Core Matters: Results that Yield Successful Business Outcomes & Substantial Compliance
We’re passionate about figuring out how to expertly integrate both the intent and interpretation of regulations to your specific products. So ask yourself how [...]
BioTeknica at Upcoming Conferences: 2015 – 2016
BioTeknica at Upcoming Conferences: 2015 – 2016 BioTeknica will be participating at the following upcoming conferences. We look forward to seeing you there! 10th [...]