Critical Issue Management
Critical Issue Management for FDA 483 Warning Letters, Recalls & Safety Alerts Agency Representation & Negotiation Recalls, Field Corrections & Safety Alerts 483/Warning Letter/Consent Decree Resolution Compliance Certification Import Alert & Detention Removal Programs Consent Decree Programs HIPAA Subpoenas Government Investigations Major Product's Liability Litigation Integrity Inquiries Import Alerts/Bans Seizure Injunctions Administrative Detention Civil Actions Criminal Actions Corporate or Multi-Facility Warning Letters Monitoring of North American & European Union Regulatory Activities What is Critical Issue Management? BioTeknica, an IQVIA business, can help resolve issues arising from FDA management [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory support for all phases [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare for your next FDA [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We ensure that your quality [...]
INDUSTRY NEWS ROUNDUP
Due to the COVID-19 pandemic, the Association of Food and Drug Officials (AFDO) Annual Conference, originally scheduled for June [...]
SUCCESS STORIES Bringing an Inadequate CAPA Process to Effectiveness
Did you know that one of the top three causes of 483 observations is because manufacturers fail to correctly [...]
ASK A BIOTEKNICA SME HOW TO VALIDATE A PROCESS WITH A “HIGH DEGREE OF ASSURANCE” AND WHAT DOES THAT EVEN MEAN?
Looking for answers to your regulatory questions? To submit one, please click on Ask a BioTeknica SME and our Subject Matter [...]
ASR IS OVER! HOW DO VMSR & NEST IMPACT YOUR ORGANIZATION?
Feeling unsure of how the FDA’s updated reporting processes will affect you? You’re not alone. BioTeknica SMEs delved into [...]
Critical Issue Management
Critical Issue Management for FDA 483 Warning Letters, Recalls & Safety Alerts Agency Representation & [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We [...]
Ready, Set, Go! Coaching Your Team to Validation Success
Ready, Set, Go! Coaching Your Team to Validation Success Device manufacturers often don’t have the time or the internal resources to train their [...]
Get to the Head of the Class: Lunch & Learn Series at BioTeknica’s New Learning Center
Get to the Head of the Class: Lunch & Learn Series at BioTeknica's New Learning Center BioTeknica recently launched Lunch & Learn, a [...]
Julie Larsen Joins FDANews Medical Device Conference Advisory Board
Julie Larsen Joins FDANews Medical Device Conference Advisory Board Congratulations to BioTeknica principal Julie Larsen for her recent invitation and acceptance to join [...]
Charity: BioTeknica’s Helping Hands
Charity: BioTeknica's Helping Hands Every year for the past three and a half years, BioTeknica has partnered with Catholic Relief Services (CRS) to support Helping [...]
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
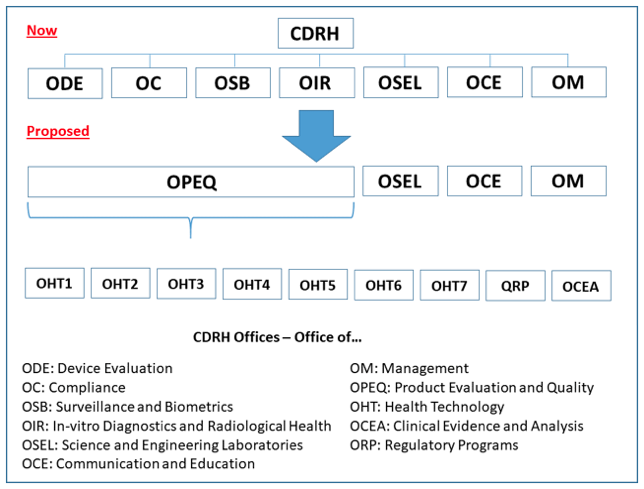
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health (CDRH) has submitted a reorganization plan [...]
FDA’s Office Of Regulatory Affairs Realigned
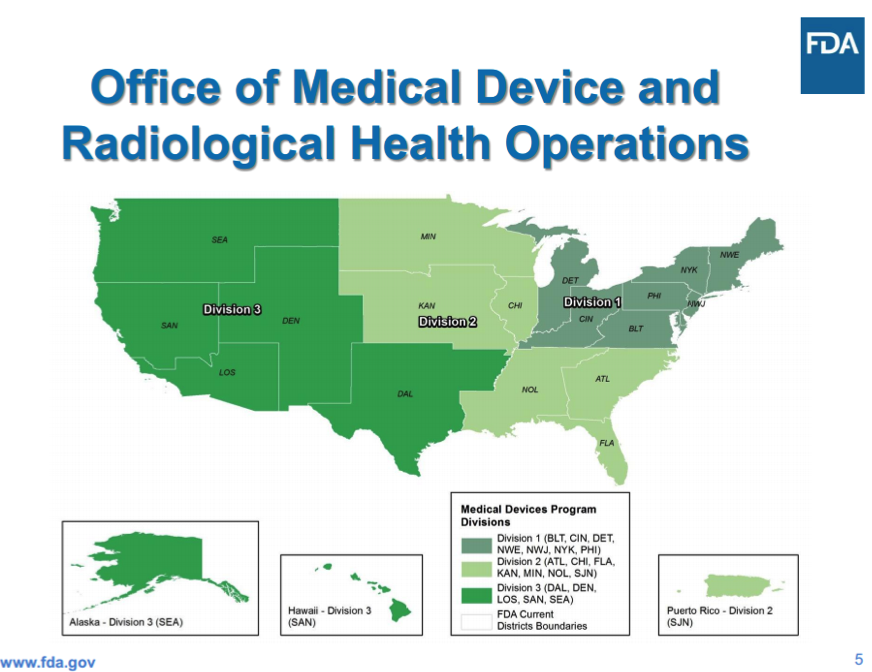
FDA's Office Of Regulatory Affairs Realigned The FDA’s Office of Regulatory Affairs (ORA), the lead office for agency field activities, has changed its [...]