IS/IT Information Systems Management
IS/ IT Information Systems Management A Division of BioTeknica, an IQVIA Business About Our Information Systems Management Services Access to the right data at the right time is the key to making Quality and Compliance business decisions. QDS brings together a comprehensive portfolio of Business Intelligence and Data Management services, software, and expertise to deliver risk transparency across an organization’s Quality Management Systems and related business processes. In today’s fast-paced business environments, an organization's ability to satisfy its client's needs are directly related to their ability to process data and make sound decisions. In order to protect your investment in quality data, our team provides Information Management Services that incorporate People, Process, and Technology. [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & Positively Impacting Your Bottom [...]
Quality Engineering
Quality Engineering Are you able to resolve the root cause? Quality Engineering services using proven methodologies to [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We ensure that [...]
Lean Quality Solutions
Lean Quality Solutions for Medical Device & Pharmaceutical Companies Synergis, a sister company of BioTeknica, an IQVIA business, [...]
BIOTEKNICA COMMUNITY OUTREACH
BIOTEKNICA COMMUNITY OUTREACH Peru, Miami, Africa! When not traveling around the world for client business, BioTeknica team members can [...]
TEAM MEMBER PROFILE Meet Ruben Capo: Co-Founder, Quality Engineer, Part-Time Adventurer
Meet Ruben, mild-mannered BioTeknica Principal, Co-Founder, and Senior Quality Engineer. Think you know him? Think again. When you [...]
IS/IT Information Systems Management
IS/ IT Information Systems Management A Division of BioTeknica, an IQVIA Business About Our Information Systems Management Services [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & [...]
Quality Engineering
Quality Engineering Are you able to resolve the root cause? Quality Engineering services [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for [...]
KEY FDA EXPECTATIONS FOR ADVERSE EVENT REPORTING DURING A PANDEMIC
The FDA has modified the regulations regarding adverse event reporting during a pandemic to include COVID-19. Click below for key points about the updated [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open and operating at full capacity with the [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact the device industry: [...]
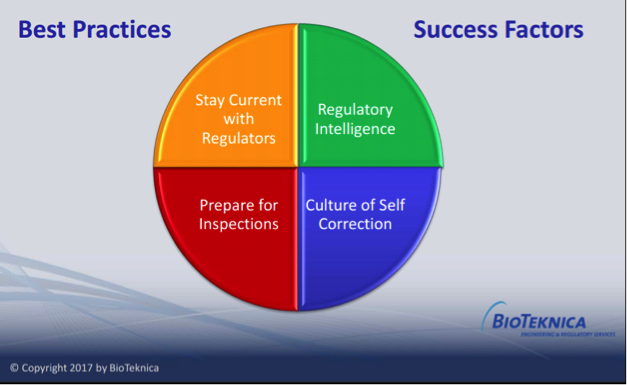
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American Society for Quality (ASQ) conference [...]
Validation: When is a Test Method Not a Test Method?
Validation: When is a Test Method Not a Test Method? How can one site conduct and pass several gage Repeatability and Reproducibility (R&R) [...]
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015 BioTeknica has successfully achieved ISO 9001:2015 certification, the highest standards of its kind, for its Quality Management (QM) [...]