S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & Positively Impacting Your Bottom Line? YES… when you follow our S3 – Simple, Systematic, Sustainable methods! Simple, Systematic, Sustainable Methods for Regulatory Services Our proven methods provide a strategically designed approach to regulatory services: Simple – straight forward compliant solutions that are easy to understand and execute Systematic – solutions that are integrated rather than patch-worked and disjointed Sustainable – practical solutions that are reliable, mistake-proof and self flagging, thereby requiring little overhead to maintain as part of an integrated Quality System
TEAM MEMBER PROFILE Meet Ruben Capo: Co-Founder, Quality Engineer, Part-Time Adventurer
Meet Ruben, mild-mannered BioTeknica Principal, Co-Founder, and Senior Quality Engineer. Think you know him? Think again. When you talk to [...]
INDUSTRY NEWS ROUNDUP
Due to the COVID-19 pandemic, the Association of Food and Drug Officials (AFDO) Annual Conference, originally scheduled for June 2020 in [...]
SUCCESS STORIES Bringing an Inadequate CAPA Process to Effectiveness
Did you know that one of the top three causes of 483 observations is because manufacturers fail to correctly use their [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws [...]
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American [...]
Validation: When is a Test Method Not a Test Method?
Validation: When is a Test Method Not a Test Method? How can one site conduct and pass several [...]
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015
BioTeknica Awarded Highest Standards Certification: ISO 9001:2015 BioTeknica has successfully achieved ISO 9001:2015 certification, the highest standards of its kind, [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & Positively Impacting Your Bottom Line? [...]
TEAM MEMBER PROFILE Meet Ruben Capo: Co-Founder, Quality Engineer, Part-Time Adventurer
Meet Ruben, mild-mannered BioTeknica Principal, Co-Founder, and Senior Quality Engineer. Think you know him? Think again. [...]
INDUSTRY NEWS ROUNDUP
Due to the COVID-19 pandemic, the Association of Food and Drug Officials (AFDO) Annual Conference, originally scheduled [...]
SUCCESS STORIES Bringing an Inadequate CAPA Process to Effectiveness
Did you know that one of the top three causes of 483 observations is because manufacturers fail [...]
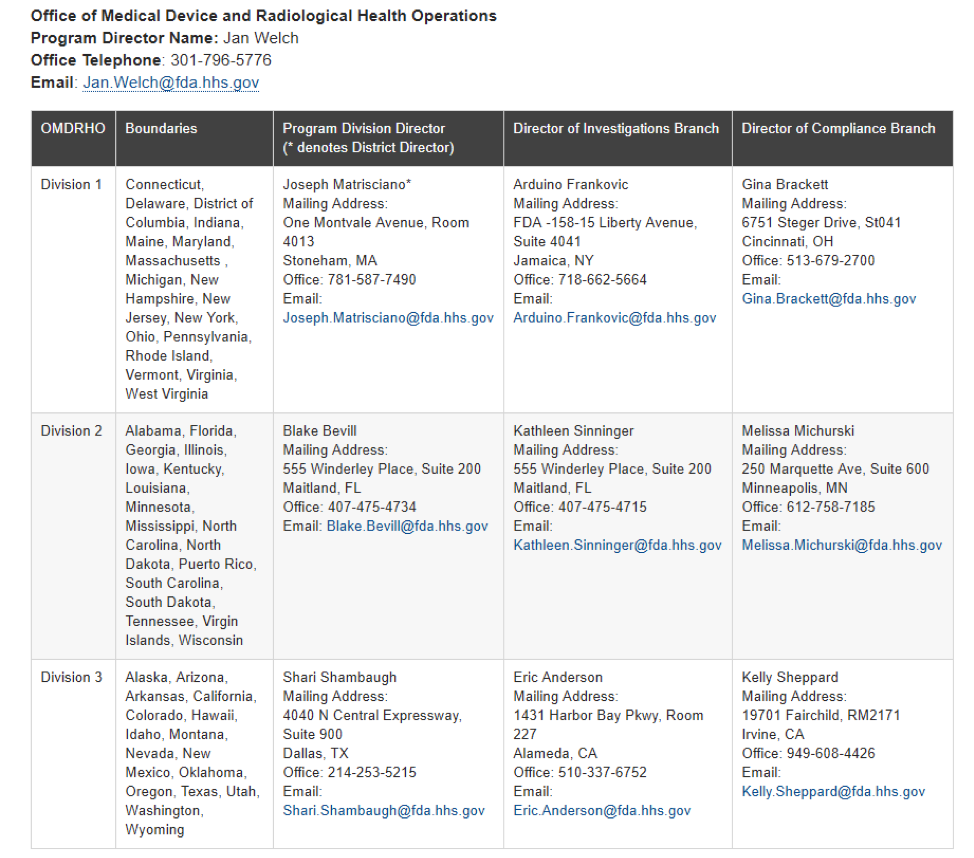
New FDA Communications Guidelines For Inspections
New FDA Communications Guidelines For Inspections Medical device companies have new FDA contacts and new guidelines to communicate with the FDA regarding their [...]
Modernizing the FDA Workforce
Modernizing the FDA Workforce A strategic priority identified by the FDA earlier this year is to strengthen the FDA’s scientific workforce by investing [...]
BioTeknica is an ISO 9001:2015 Certified Company
BioTeknica is an ISO 9001:2015 Certified Company BioTeknica has successfully achieved ISO 9001:2015 certification, the highest standards of its kind, for its Quality [...]
Lean Management Systems and the New Normal
Lean Management Systems and the New Normal Miguel E Guerrero, Lean Sensei, Managing Partner, Synergis Corp., a BioTeknica sister company The output of any [...]
FDA Program Alignment & The New CDRH in Bite-Sized Chunks
FDA Program Alignment & The New CDRH in Bite-Sized Chunks Changes at the FDA are impacting life science manufacturers. In 2013 the Agency [...]
ISO 13485: WHAT’S NEW? FIRST REVISION IN 13 YEARS
ISO 13485: What’s New? First Revision in 13 Years It’s been 13 years since ISO 13485 was last updated, and there was a [...]