FEATURED POST
Quality Engineering
Are you able to resolve the root cause?
Quality Engineering services using proven methodologies to resolve life science engineering challenges
Operational Review
Operational Review & Optimization for Medical Device and Pharmaceutical Companies Cost Reduction Strategies Business Process Re-engineering (BPR) Process Optimization/Improvement Process Control [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & Positively Impacting Your Bottom [...]
Quality Engineering
Quality Engineering Are you able to resolve the root cause? Quality Engineering services using proven methodologies to [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare for your [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We ensure that [...]
Lean Quality Solutions
Lean Quality Solutions for Medical Device & Pharmaceutical Companies Synergis, a sister company of BioTeknica, an IQVIA business, [...]
BIOTEKNICA COMMUNITY OUTREACH
BIOTEKNICA COMMUNITY OUTREACH Peru, Miami, Africa! When not traveling around the world for client business, BioTeknica team members can [...]
Due Diligence and Quality Systems Integration… More Important than Ever
Due Diligence and Quality Systems Integration… More Important than Ever during Tumultuous Period for Pharmaceutical and Medical Device Industry Global Trade Wars … Supply [...]
Operational Review
Operational Review & Optimization for Medical Device and Pharmaceutical Companies Cost Reduction Strategies Business Process Re-engineering (BPR) [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & [...]
Quality Engineering
Quality Engineering Are you able to resolve the root cause? Quality Engineering services [...]
ASR IS OVER! HOW DO VMSR & NEST IMPACT YOUR ORGANIZATION?
Feeling unsure of how the FDA’s updated reporting processes will affect you? You’re not alone. BioTeknica SMEs delved into the real-world impact of these [...]
KEY FDA EXPECTATIONS FOR ADVERSE EVENT REPORTING DURING A PANDEMIC
The FDA has modified the regulations regarding adverse event reporting during a pandemic to include COVID-19. Click below for key points about the updated [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open and operating at full capacity with the [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact the device industry: [...]
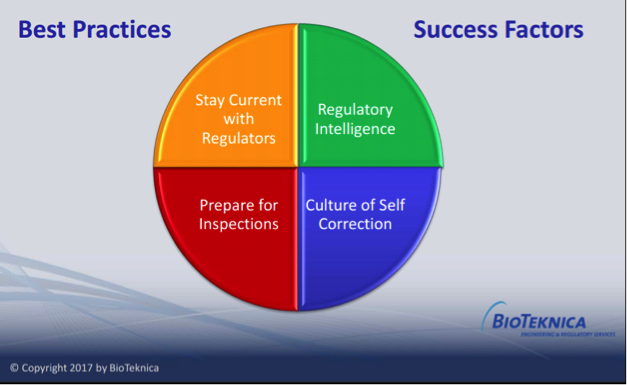
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American Society for Quality (ASQ) conference [...]
Validation: When is a Test Method Not a Test Method?
Validation: When is a Test Method Not a Test Method? How can one site conduct and pass several gage Repeatability and Reproducibility (R&R) [...]