The FDA has modified the regulations regarding adverse event reporting during a pandemic to include COVID-19. Click below for key points about the updated guidance as of March 2020.
Please be assured that in the event of further disruptions, we are prepared to continue with our services remotely in a secure environment for our employees and customers.
The COVID-19 pandemic has interrupted our lives and our organizations in so many unimaginable ways. However, one segment of our business processes will remain the same; the FDA is expecting life science manufacturers to maintain normal adverse event reporting processes “to the maximum extent possible” during the COVID-19 pandemic. According to the agency:
- Use your organization’s standard operating procedures to handle all adverse event data
- Regulatory and statutory requirements for adverse events should be met to the maximum extent possible
- If not able to fulfill all adverse event reporting requirements because of high employee absenteeism caused by the pandemic, your organization should develop and prepare to implement its Continuity of Operations Program (COOP)
- The agency will understand if, because of pandemic-related high employee absenteeism, certain adverse event reports are not submitted to the FDA within the timeframes required by statute and regulation, as long as any delayed reports are submitted within 6 months of the restoration of adverse event reporting processes to their pre-pandemic state
- Types of things to consider:
- What activities are directly relevant to the processing and submission of mandatory adverse event reports to FDA?
- How would sites based in the United States and abroad be differentially affected by a pandemic?
- What are the relative amounts of resources dedicated to mandatory adverse event reporting at each site?
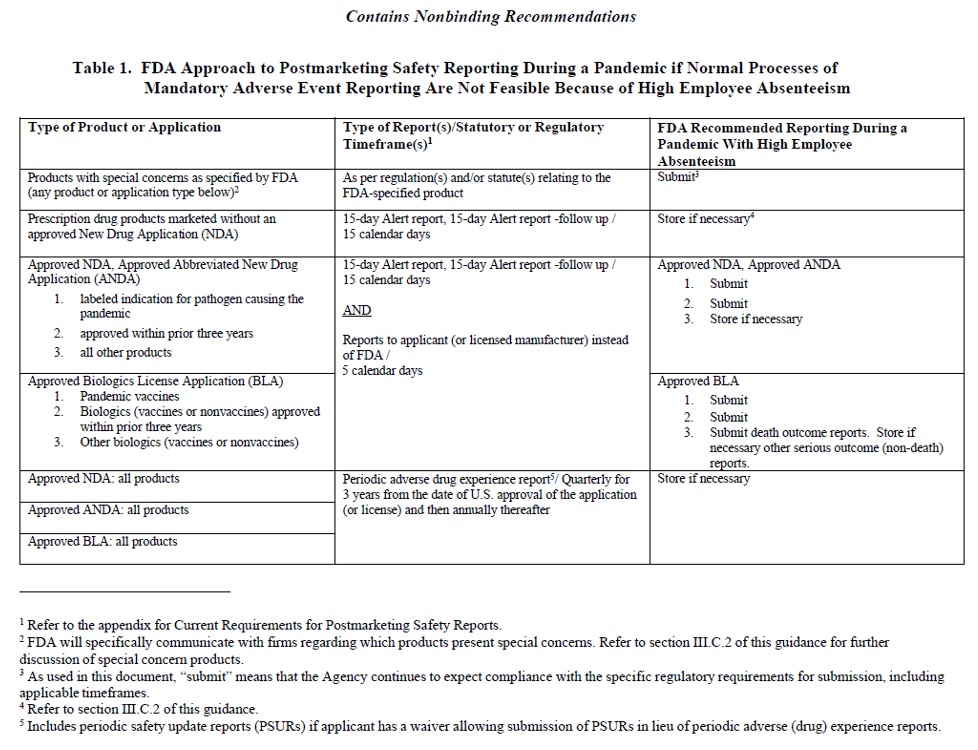
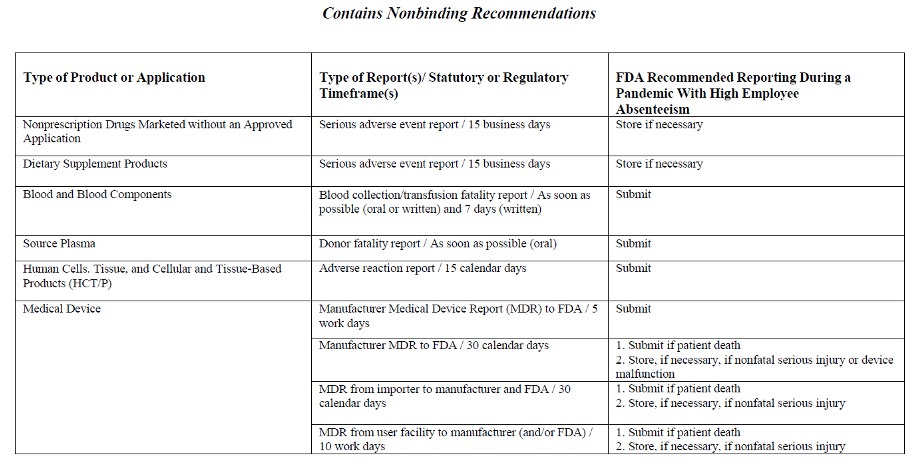
Below are two tables from the FDA, as of March 2020, that reflect the timetables for post-marketing safety reporting during a pandemic when and/or if normal processes of mandatory reporting are not feasible because of high employee absenteeism. Click on the link below to access the final guidance document.
FDA Final Guidance Document for Medical Device Post Marketing Adverse Event Reporting



Leave A Comment