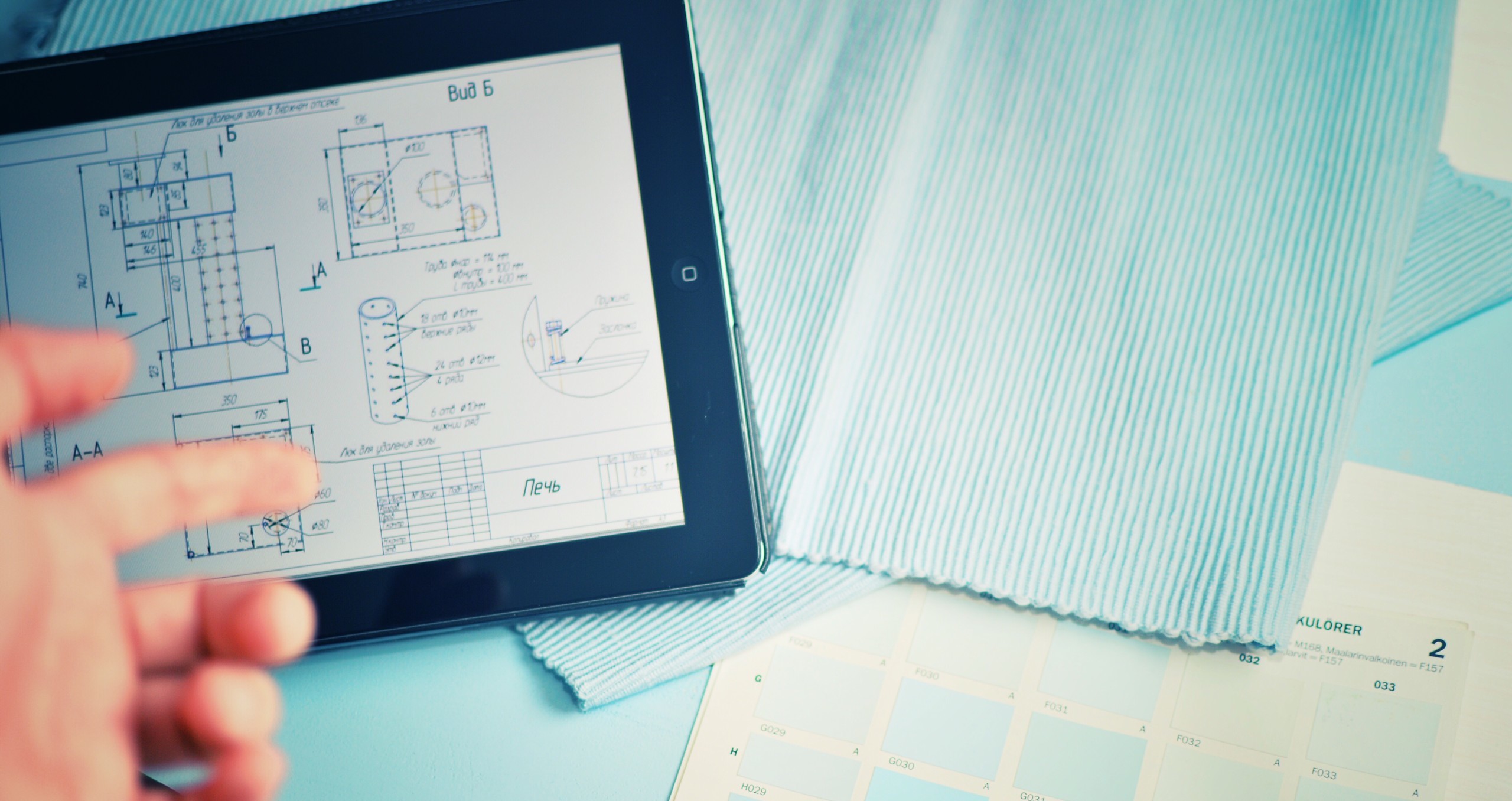
The Importance of Process Validation: Lessons from Recent FDA Warning Letters
How Non-Compliance Can Impact Medical Device Manufacturers—and What You Can Do to Prepare Process validation is a critical requirement for medical device manufacturers. The FDA routinely issues Form 483s and Warning Letters when companies fall short in this area, underscoring the importance of robust validation protocols. Since 2020, there have been at least 43 documented instances where the FDA cited non-compliance with a key regulatory requirement: “Failure to validate a process whose results cannot be fully verified by subsequent inspection and test, as required by 21 CFR §820.75(a).” Recent Examples Highlight Common Validation Pitfalls Here are two examples of common validation pitfalls we see highlighted in Warning Letters. In the first, a manufacturer of diagnostic devices received a Warning Letter after an FDA inspection [...]
The Importance of Process Validation: Lessons from Recent FDA Warning Letters
How Non-Compliance Can Impact Medical Device Manufacturers—and What You Can Do to Prepare Process validation is a critical requirement for medical device [...]
Due Diligence and Quality Systems Integration… More Important than Ever
Due Diligence and Quality Systems Integration… More Important than Ever during Tumultuous Period for Pharmaceutical and Medical Device Industry Global Trade Wars [...]
IS/IT Information Systems Management
IS/ IT Information Systems Management A Division of BioTeknica, an IQVIA Business About Our Information Systems [...]
Critical Issue Management
Critical Issue Management for FDA 483 Warning Letters, Recalls & Safety Alerts [...]
Technical Development Service
Medical Device Design Control, Product Development & Quality Testing Product Development Management Design Control Process/Design and Implementation Regulatory Compliance Regulatory, [...]
Operational Review
Operational Review & Optimization for Medical Device and Pharmaceutical Companies Cost Reduction Strategies Business Process Re-engineering (BPR) Process Optimization/Improvement [...]
S3 Method – Simple, Systematic, Sustainable
S3 Method: Simple, Systematic, Sustainable Can You Be Compliant While Keeping Costs Down & Positively Impacting [...]
The Importance of Process Validation: Lessons from Recent FDA Warning Letters
How Non-Compliance Can Impact Medical Device Manufacturers—and What You Can Do to Prepare Process validation is a critical requirement for medical device manufacturers. The [...]
The Importance of Process Validation: Lessons from Recent FDA Warning Letters
How Non-Compliance Can Impact Medical Device Manufacturers—and What You Can Do to Prepare Process validation is a critical [...]
Due Diligence and Quality Systems Integration… More Important than Ever
Due Diligence and Quality Systems Integration… More Important than Ever during Tumultuous Period for Pharmaceutical and Medical Device [...]
IS/IT Information Systems Management
IS/ IT Information Systems Management A Division of BioTeknica, an IQVIA Business [...]
Quality Engineering
Quality Engineering Are you able to resolve the root cause? Quality Engineering services using proven methodologies to resolve life science [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both small and large [...]
Regulatory Services
Regulatory Services for Medical Device & Pharmaceutical Industries BioTeknica provides expert clinical and regulatory support for all phases of global medical [...]
FDA Inspection Readiness
S3 FDA Inspection Readiness Services Are You Ready? Have you done everything possible to prepare for your next FDA Inspection? [...]
Regulatory Compliance
Regulatory Compliance We not only know what to do but – how to do it. We ensure that your quality systems are compliant [...]
Lean Quality Solutions
Lean Quality Solutions for Medical Device & Pharmaceutical Companies Synergis, a sister company of BioTeknica, an IQVIA business, offers comprehensive lean quality solutions. [...]