New FDA Communications Guidelines For Inspections
Medical device companies have new FDA contacts and new guidelines to communicate with the FDA regarding their inspections. Find your division and contacts and learn more about the guidelines.
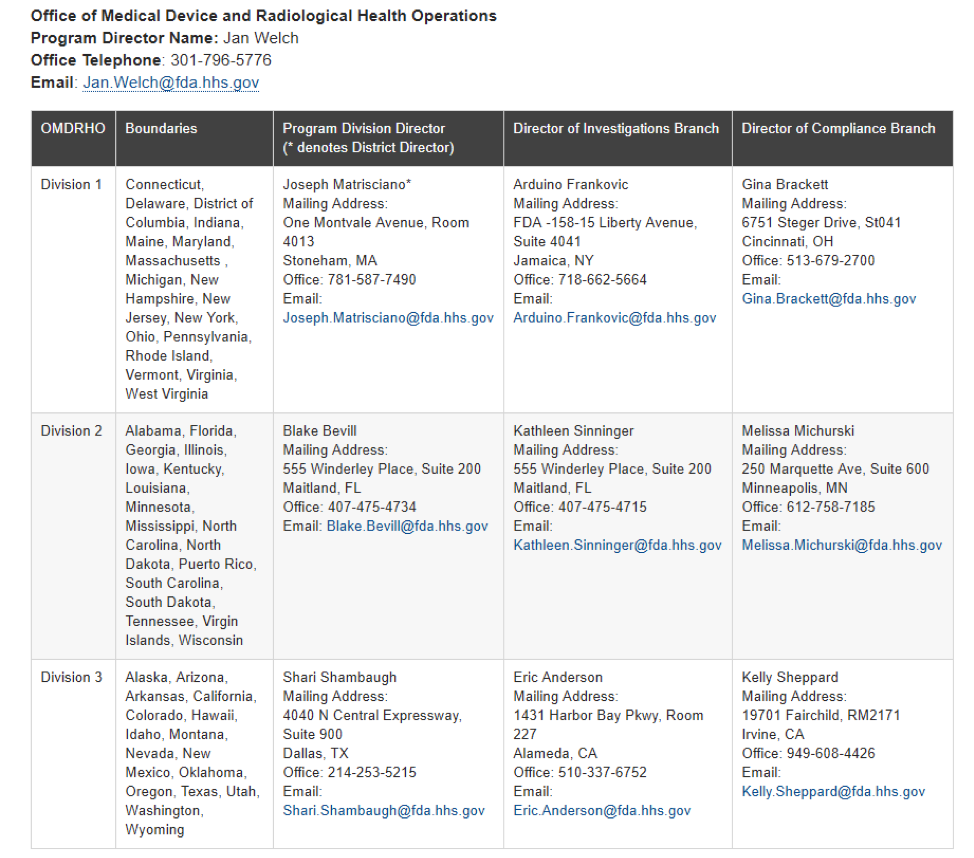
The Office of Medical Device and Radiological Health Operations (OMDRHO) provides advice to the Office of Regulatory Affairs (ORA) and collaborates with the Center for Devices and Radiological Health (CDRH) regarding devices and radiation-emitting products, coordinates and directs inspection activities, and provides technical assistance for inspectional operations.
Below is a chart that outlines the geographic regions of each boundary and the contact information for their corresponding sets of directors.
- The program Division Director supervises all inspections and compliance activities.
- The Director of Investigations Branch manages all inspectional activities.
- The Director of Compliance Branch manages responses and post-inspection compliance activities

The best way to correspond with the OMDRHO is via email, depending on your Division. In a presentation from FDA official Blake Bevill earlier this year, device companies should contact the following individuals regarding FDA inspections- and recall-related correspondence.
Division 1 – East
- FDA inspection-related correspondence – ORAdevices1firmresponse@fda.hhs.gov
- Recall-related correspondence – ORAdevices1recalls@fda.hhs.gov
- FDA Administrative Officer – Donna Dismukes – Donna.Dismukes@fda.hhs.gov or 787-587-7451
Send CDs and Thumb Drives to:
U.S. Food & Drug Administration
Office of Medical Device & Radiological Health Operations
Division 1 East
ATTN: OMDRHO Div 1 Correspondence
One Montvale Ave.
Stoneham, MA 02180
Division 2 – Central
- FDA inspection-related correspondence – ORAdevices2firmresponse@fda.hhs.gov
- Recall-related correspondence and calls – ORAdevices2recalls@fda.hhs.gov
Neisa Alonso – 407-475-4717
Meredith Andress – 407-475-4722
Marie Fink – 504-846-6109
Send CDs and Thumb Drives to:
U.S. Food & Drug Administration
Office of Medical Device & Radiological Health Operations
Division 2 Central
ATTN: OMDRHO Program Division Director
555 Winderley Place
Suite 200
Maitland, FL 32751
Division 3 – West
- FDA inspection-related correspondence – ORAdevices3firmresponse@fda.hhs.gov
- Recall-related correspondence and calls – ORAdevices3recalls@fda.hhs.gov
Theresa Kirkham – 949-608-4437
Send CDs and Thumb Drives to:
U.S. Food & Drug Administration
Office of Medical Device & Radiological Health Operations
Division 3 West
ATTN: Program Division Director
19701 Fairchild
Irvine, CA 92612
The OMDRHO requests that any PDF attachments should not exceed 100MB per file. If you need to send CDs or thumb drives, please send via regular mail to the appropriate division office.



Leave A Comment