5 Things You Should Know from the 2015 Association of Food & Drug Officials (AFDO) Conference
If you didn’t have a chance to attend this year’s AFDO Annual Educational Conference, below are five important take-away points from the Drugs, Devices and Cosmetics sessions.
1. EU Enforcement: Notified Bodies Now Conducting Unannounced Audits of Manufacturers, Sub-Contractors & Suppliers

The FDA can appear at your doorstep without warning, and now, the European Notified Bodies can also show up unexpectedly. Competent Authorities are now assessing Notified Bodies and the onus is on the Notified Bodies to conduct more unannounced audits of manufacturers. As a result of these new regulations, manufacturers across the European Union (EU), including some of BioTeknica’s clients, have already experienced audits that were unannounced.
Life science companies selling products in the EU should be aware of two key regulations that became effective in January 2014:
- EU No 920/2013, which directs Competent Authorities on how to control Notified Bodies, and
- 2013/473/EU, which directs Notified Bodies on how to audit manufacturers
So what do you need to know?
- Who can receive an unannounced audit? Manufacturers as well as their critical sub-contractors, crucial suppliers and contract manufacturers are all susceptible to these unannounced visits. The responsible legal entity, the finished goods manufacturer, will be notified and invited to attend the audit’s closing meeting. Audit reports will also go to the manufacturer.
- How often will unannounced audits occur? They will occur at least once every three years and will be more frequent for companies with a history of noncompliance or higher risk products.
- What’s the procedure for an audit? Typically, the audits will not follow a set agenda; they can last one to two days; and involve two assessors who will likely not be the same assessors routinely seen by the company.
- The normal elements will not be reviewed, but will include any two of the following critical processes: Design Control, Establishment of material specifications, purchasing and control of incoming material, assembling, sterilization, batch release, packaging, product quality control
- Product audits are included to confirm compliance to the technical files
- Can a Manufacturer or its suppliers and contractors refuse an audit? Should the company refuse to allow an audit, it could result in scope reduction, suspension or cancellation of a certificate and even loss of a CE Mark. You should develop a strategy to determine how you can protect your company from a supplier or contractor who refuses an audit. BioTeknica can assist in developing strategies for your specific products and suppliers.
Do you have questions or comments about EU Enforcement?
2. New Device Correction Guidance: Is it a Recall or an Enhancement? FDA Expects You to Know

While the FDA has always permitted manufacturers to make enhancements to devices, there was no clear guidance. Today, the agency’s guidance clarifies the differences between device corrections and enhancements and clarifies reporting requirements under 21 CFR Part 806. The FDA released written guidance in October 2014 on how the agency expects device manufacturers to distinguish product enhancements from device recalls.
What does this mean for device manufacturers?
- Companies will need to understand and define the differences between a recall and an enhancement.
- Investigators will expect to see records of device enhancements.
- Manufacturers will have to decide how to best document their product enhancement changes.
- This could impact any upcoming FDA inspections.
The challenge is that the FDA is not providing specific instructions on how or where they expect the recall or enhancement decisions to be documented. BioTeknica’s Julie Larsen and her colleagues can discuss how this can specifically impact your products.
Do you have questions or comments about the New Device Correction Guidance?
3. Compliance Panel: Your Questions Answered
During the AFDO conference, industry representatives like you had the rare opportunity to have their compliance questions answered about the application and interpretation of regulations as applied to their own specific products. Answers were provided by a distinguished panel of FDA decision-makers. Ten questions were covered in-depth during the session. Below are three general, but key points from the FDA panel:
- Fixing vs. Enhancing Systems. FDA made it clear that if a company has a significant compliance issue, then the company cannot state that it is “enhancing” its system, but instead is “fixing” the system.
- Complete Complaint Investigations. Given that many products are often not returned, when conducting a complaint investigation, manufacturers are expected to go beyond just the review of a returned product, and instead are expected to “Look at all of your sources of information.”
- MDR Dating. The date that should be used for the Medical Device Reporting (MDR) is the date that the company became aware (usually initial complaint notification) of an adverse event. It is not acceptable to use the date on which you received a returned product.
BioTeknica’s Julie Larsen moderated the session, which consisted of FDA panelists:
- Ricki Chase, Director of Investigations, Chicago District
- Maureen Bernier, Biomedical Engineer/Recall Coordinator, Center for Devices and Radiological Health
- Steven Barber, Director Compliance Branch, Detroit District
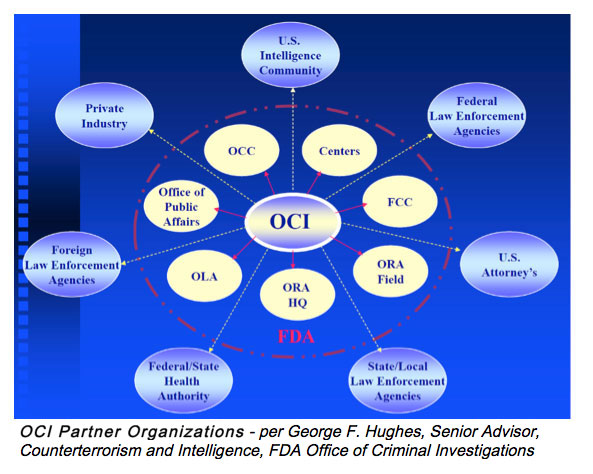
- George Hughes, Office of Criminal Investigations.
Do you have questions or comments for next year’s AFDO Compliance Panel?
4. Medical Device Component Counterfeiting
George F. Hughes, Senior Advisor Counterterrorism and Intelligence, FDA Office of Criminal Investigations, conveyed how his office partners with industry to address counterfeit issues. An industry representative shared an example of a counterfeit material that resulted in a recall for their company. The importance of establishing controls to confirm authenticity of critical components at the point of receipt was discussed.
5. Questions on How These Regulations Impact Your Specific Products?
For the past several years, Julie Larsen, Principal and Director, Inspection Readiness Services, BioTeknica, has been an organizer and moderator for the AFDO Annual Educational Conference. She serves on the AFDO Drugs, Devices and Cosmetics Committee, working with FDA decision-makers and other experts to develop the agenda and plan conference activities for this track. She has served as an expert panelist at various conferences where she provides guidance and advice on regulatory inspection challenges. Feel free to contact BioTeknica, where Julie or her colleagues can help you with any questions.



Leave A Comment