FEATURED POST
What You Can Learn from 7 Theme Fusion Success Stories
Nam lacinia arcu tortor, nec luctus nibh dignissim eu. Nulla sit amet maximus nulla. Pellentesque a accumsan eros, ac molestie nulla. Morbi interdum in neque vitae vulputate.
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open and operating at full [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two recent laws will impact [...]
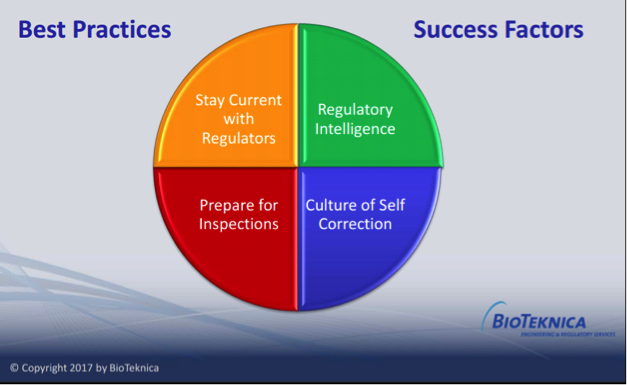
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of the American Society for [...]
Promotion Announcement
Promotion Announcement We are pleased to announce the promotion of several BioTeknica team members. Please join us in [...]
Charity: BioTeknica’s Helping Hands
Charity: BioTeknica's Helping Hands Every year for the past three and a half years, BioTeknica has partnered with Catholic Relief [...]
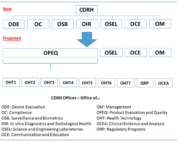
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency
FDA Program Realignment: New Model for Device Approval & Inspection Efficiency The FDA Center for Devices and Radiological Health [...]
FDA’s Office Of Regulatory Affairs Realigned
FDA's Office Of Regulatory Affairs Realigned The FDA’s Office of Regulatory Affairs (ORA), the lead office for agency [...]
Validation
Validation Services BioTeknica, an IQVIA business, provides proven validation methodologies that have been successfully implemented for our clients at both small and [...]
BIOTEKNICA OPERATING AT FULL CAPACITY Company Implements Business Continuity Plan During COVID-19 Crisis
BioTeknica and its sister companies, Qualified Data Systems and Synergis, are here for you. We are open [...]
2 New Laws Impact Device Industry
2 New Laws Impact Device Industry According to a presentation by FDA official Blake Bevill, two [...]
4 Strategies for Keeping Current In An Evolving World of Compliance
4 Strategies for Keeping Current In An Evolving World of Compliance At a half-day conference of [...]
Successful Outcomes One Client at a Time
Successful Outcomes One Client at a Time Recently, BioTeknica assisted a leading medical device manufacturer to successfully address a previous FDA Warning Letter by [...]
Core Matters: Results that Yield Successful Business Outcomes & Substantial Compliance
We’re passionate about figuring out how to expertly integrate both the intent and interpretation of regulations to your specific products. So ask yourself how [...]
BioTeknica at Upcoming Conferences: 2015 – 2016
BioTeknica at Upcoming Conferences: 2015 – 2016 BioTeknica will be participating at the following upcoming conferences. We look forward to seeing you there! 10th [...]